Dear readers,
here we are with another useful website to obtain restriction maps in a click!
http://www.geneinfinity.org/sms/sms_remap.html
Restriction enzymes cut DNA in specific sites according to the nucleotide sequences that they recognize.
A restriction map shows all the possible cuts depending on the enzyme used.
The website is easy to use. We just need to copy our DNA sequence (in Fasta format) and submit.
Good luck and I'll be back soon! :)
venerdì 22 luglio 2011
mercoledì 25 maggio 2011
Bioinformatics - Useful websites
Dear beautiful people,
Are you interested to compare genomes?
Dotlet: http://myhits.isb-sib.ch/cgi-bin/dotlet
Are you interested to protein structures?
Swiss-PdbViewer: http://spdbv.vital-it.ch/ (free download)
Application that provides a user friendly interface allowing to analyze several proteins at the same time. This software is able to read file.pdb (file containing protein structures).
Prosa: https://prosa.services.came.sbg.ac.at/prosa.php
With PDB files of our protein we can get an energetic graph based on the structure.
VADAR: http://vadar.wishartlab.com/
For analyzing and assessing peptide and protein structures from their PDB coordinate data.
It's bioinformatic time! :)
 |
| Prof. Jean C. Shih (USC Neuroscience) - I also want a pic like this! |
First of all let's become familiar with Protein Data Bank (http://www.rcsb.org/pdb/home/home.do), the best website ever to collect informations about our proteins. Here you can find DNA sequences generally in fasta format files, and structures in PDB files.
Are you interested to compare genomes?
Here two easy softwares:
Dotlet: http://myhits.isb-sib.ch/cgi-bin/dotlet
EMBOSS: http://www.ebi.ac.uk/Tools/psa/emboss_stretcher/
Are you interested to protein structures?
Swiss-PdbViewer: http://spdbv.vital-it.ch/ (free download)
Application that provides a user friendly interface allowing to analyze several proteins at the same time. This software is able to read file.pdb (file containing protein structures).
Prosa: https://prosa.services.came.sbg.ac.at/prosa.php
With PDB files of our protein we can get an energetic graph based on the structure.
VADAR: http://vadar.wishartlab.com/
For analyzing and assessing peptide and protein structures from their PDB coordinate data.
P.s. In my previous post i told you about the resazurin problem with the HPLC-MS, well the solution was even easier than I thought. I'm not using it in my samples but only in some control bottle that will not be analysed. Of course according to me this is not such a scientific method but the controls don't show oxygen presence for the moment and my bacteria are growing so well!!! :):):)
Au revoir!
giovedì 31 marzo 2011
Thesis - technical hitches :S
Beautiful folk! :)
Here I am with the first problems with my thesis...
I'm actually preparing the colture medium for my anaerobic bacteria, of course to be sure that no oxygen is inside i need a redox indicator. In my previous post i mentioned Resazurin, apparently this compound create problems to our HPLC-MS releasing crystals in the columns. I should find a way to take off the Resazurin before the analysis... not so easy considering that I'm monitoring PFCs (PFOS, PFOA and PFBS) and the structure of my redox indicator doesn't allow me to use a lot of extraction techniques without damaging my metabolites! :(
Checking also other redox indicators i could notice how they are really similar between them...
In conclusion i thought to use Titanium(III)-citrate that becomes white once oxydated. Of course i should take into account in my final calculations that it could degradate my PFCs as shown in the following article: http://www.mendeley.com/research/reductive-defluorination-of-perfluorooctane-sulfonate/# ...
I'm going to propose this to my supervisor and I'll let you know! :):):)
Besitooos!
martedì 1 marzo 2011
Best Pasta Ever (BPE) recipe! :)
Ladies and gentlemen,
welcome to "Fra in da kitchen" lesson one! :)
I'll try to explain you how to prepare the pasta that has been considered the best ever by my friends Party Boy and The Douche. I know a lot of celebrities... eh eh eh!
Here we are schematic and painless:
(the recipe is for 4-5 persons, Party Boy and The Douche can finish the same amount alone! :S)
(the recipe is for 4-5 persons, Party Boy and The Douche can finish the same amount alone! :S)
- Oil in the pan with 250g of mushrooms, well cut (better champignon)
- After couple of mins add bacon (figure 1)
- When the bacon is ready add peas (figure 2)
- In 5 mins our sauce is ready.
- Boil the water and add 3 spoons of salt.
- Add the pasta (500 g) when the water is boiling, and mix it.
- Wait 12 mins for "fusilli" or "penne".
- Remove the water and add the sauce and a bit of oil.
- Warm it up (over a low flame) and mix for around 5 mins.
 |
| Figure 1: I know, it's expired! |
Variations: you could add milk cream between step 8 and 9.
To conclude i would like to give you some tips to avoid bad italian restaurants, so stay away from places that offer you:
- Pizza bolognese (what's that???)
- Makkaroni, macaronni etc. (what's that again??? better if you find "Spaghetti"!)
- Pesto in sandwiches (absolutely not!)
- Waiters called Napoli/Firenze :D
Bon appetit! :)
sabato 26 febbraio 2011
Desulfitobacterium: growth medium, add salt to taste! ;)
Preparing a growth medium is as esay as following a recipe! :)
This is the colture medium I'm going to use with Desulfitobacteria hafniense DCB-2, D. hafniense TCE-1, D. chlororespirans, D. dehalogenans, D. PCE1.
This is the colture medium I'm going to use with Desulfitobacteria hafniense DCB-2, D. hafniense TCE-1, D. chlororespirans, D. dehalogenans, D. PCE1.
Pay attention to respect the right concentration and follow the protocol i've posted to prepare anaerobic solutions.
Starting solutions:
- M1= K2HPO4 (0.2 M)
- M2= NaH2PO4 * 2H2O (0.2 M)
- M3= Resazurine (0.5 g/L)
- M4= EDTA 500 (mg/L)
- M5=
- Vitamin H (50 mg/L)
- p-aminobenzoate (250 mg/L)
- Pantothenate (50 mg/L)
- Folic acid (20 mg/L)
- Lipoic acid (50 mg/L)
- Vitamin B6 (100 mg/L)
- Nicotinamide (550 mg/L)
- M6= Vitamin B1 (100 mg/L)
- M7= Vitamin B2 (50 mg/L)
- M8= Vitamin B12 (50 mg/L)
- M9= NH4HCO3 (114 mM) + NaHCO3 (906 mM)
- M10= Na2S * 9H2O (1M)
- M11= CaCl2 * H2O (30 mM) + MgCl2 * 6 H2O (20 mM)
- M12= Yeast extract (4%)
- M13= Na2SeO3 (6 mg/L)
All solutions were made in anaerobic water and flushed with N2 (except for M9 and M10 which contain volatile compounds).
Basic medium
- 21 ml M1
- 7 ml M2
- 1 ml M3
- 971 ml “sub-boiled” water
Cold solutions
- Cold A: in anaerobic, sterile bottle with 20 ml sterile demi-water added were: 1 ml M4, 1 ml M5, 1 ml M6, 1 ml M7, 1 ml M8, 0,5 ml M13.
- Cold B: 1 ml M10, 49 ml M9 filter-sterilised.
- Cold C: ~30 ml M11.
Completing the growing medium
To 18 ml basic medium (M1+M2+M3) add:
- 1 ml Cold B
- 0.5 ml Cold A
- 0.5 ml Cold C
- 1 ml 4% yeast extract
- Inoculum (10%)
 |
| Desulfitobacteria hafiense DCB-2 (University of California) |
So calculators in hand and good luck!
Soon to your screen the Best Pasta Ever recipe! :D
Soon to your screen the Best Pasta Ever recipe! :D
mercoledì 23 febbraio 2011
Standard Addition, the escape route! ;)
Tired of Internal and External Standard?
There is always a third solution... Standard Addition! :)
Quantification with Standard Addition is often used when the matrix of the sample can significantly modify the analytical sensitivity of the measurements.
I've performed this procedure with dust samples(dirty enough!) and LC-UV analysis.
I'll try to explain this procedure in the simplest way. :)
Let's imagine to have four samples, same volume and unknown concentration of analyte.
There is always a third solution... Standard Addition! :)
Quantification with Standard Addition is often used when the matrix of the sample can significantly modify the analytical sensitivity of the measurements.
I've performed this procedure with dust samples(dirty enough!) and LC-UV analysis.
I'll try to explain this procedure in the simplest way. :)
Let's imagine to have four samples, same volume and unknown concentration of analyte.
 |
| David L. Zellmer (1998) |
A series of increasing volumes of stock solution (solution with isotopically marked carbons) are added.
According to the literature: "The concentration and volume of the stock solution added should be chosen to increase the concentration of the unknown by about 30% in each succeeding flask".
My tip is to increase it more than 30%. I'll explain later why.
It's not necessary filling up the vials with solvents as shown in figure (in our case Vflask in the formulas is referred to our volume of sample+standard).
Just remember that this step has to be performed before the extraction, since standard solution and sample have to follow the same steps (extraction and cleaning up).
The concentration of analyte is given by:
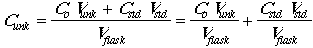
The concentration of analyte is given by:
The instrumental response of the analyte will be R = K x (concentration), so:
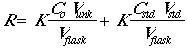
Now set Csa = CstdVstd/Vflask.
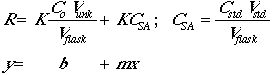
To obtain our CSA we should extrapolate for y=0, of course we need a positive value.
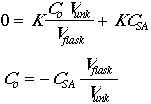
Now set Csa = CstdVstd/Vflask.
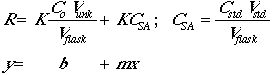
To obtain our CSA we should extrapolate for y=0, of course we need a positive value.
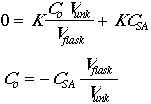
It's necessary, as for Internal and External Standard protocol, building a calibration line.
We should prepare at least six levels.
Levels are made by standard solution with different concentrations (the 1st level has standard concentration equal to zero).
They are useful to understand the real response of the machine to the different concentration.
Of course these levels go directly to the analys without any other treatment.
Plotting the pick areas, obtained from the measurement, with the concentration we should obtain a linear function.
They are useful to understand the real response of the machine to the different concentration.
Of course these levels go directly to the analys without any other treatment.
Plotting the pick areas, obtained from the measurement, with the concentration we should obtain a linear function.
The theoretical concentrations of standard solutions have to be divided by the calibration line's slope. In this way we'll obtain a new set of concentrations. Plotting these new real concentrations with the instrumental response gives us the right trend line and x-intercept, so the real analyte concentration.
As you can understand, drawing a right trend line is important to obtain the right x-intercept.
In my example the systematic error is quite big! :(
To avoid this kind of error we could:
To avoid this kind of error we could:
- add another measurement to the experiment, so that we could draw a best trend line with five points;
- choose bigger gaps between the increasing amount of standard solution, in this way we could move our points in the graph, and obtain a trend line more definite.
I think that's all folk, i hope i've been clear enough!
Good luck with Standard Addition!!! :)
venerdì 11 febbraio 2011
Glove box! Such a cool invention! :)
Supsup?
According to me the glove box, it's really a great invention, and as it often happens behind a big invention there is a simple idea.
The mechanism in fact is quite easy to understand.
As I've already explained, I'm preparing solutions without oxygen.
Of course it's not easy forbidding that oxygen spreads into our fluids, considering also that air contains 21% of this gas.
For this reason once we obtain an anaerobic solution it's always better closing the bottle with a rubber stop that has a best adhesion and does not permit to any gasses to enter.
Problems begin when is necessary working with our solutions, and maybe keeping our bottles opened for hours.
The glove box in this case results very useful. It consist in a hermetically sealed box, in which we can set the experimental conditions we need, in my case the experimental condition is "no oxygen".
Let's give an easy example.
I have to work with 24 serum bottle, with anaerobic solutions.
I'll need pipettes, tips, stoppers and of course of my bottles.
How could I put these materials into the glow box paying attention that air doesn't go inside???
Easy! :)
P.s. Glove box is also useful if we are working with toxic gasses. In this case we want that what is inside stays inside, so all the procedure described should be performed before exchanging materials from the glove box to the environment. Same thing but in the opposite way! :)
Since I'm working with anaerobic solutions i had the opportunity to meet this elegant lady that occupes a big part of my small lab.
Let me introduce you the Glove Box! :)
According to me the glove box, it's really a great invention, and as it often happens behind a big invention there is a simple idea.
The mechanism in fact is quite easy to understand.
As I've already explained, I'm preparing solutions without oxygen.
Of course it's not easy forbidding that oxygen spreads into our fluids, considering also that air contains 21% of this gas.
For this reason once we obtain an anaerobic solution it's always better closing the bottle with a rubber stop that has a best adhesion and does not permit to any gasses to enter.
Problems begin when is necessary working with our solutions, and maybe keeping our bottles opened for hours.
The glove box in this case results very useful. It consist in a hermetically sealed box, in which we can set the experimental conditions we need, in my case the experimental condition is "no oxygen".
Let's give an easy example.
I have to work with 24 serum bottle, with anaerobic solutions.
I'll need pipettes, tips, stoppers and of course of my bottles.
How could I put these materials into the glow box paying attention that air doesn't go inside???
Easy! :)
As you can see from the picture, it has an external smaller box. That's used to exchange material with the environment.
So I'll put everything into the exchanging box, I'll close it and I'll push the button connected to the vacuum pump, pressures depend by the dimension of the box, for the glow box shown in figure, 15 atm is enough.
In this way all the air contained in the little box will be carried away. Generally another system is connected to the glow box to filter and dehumidify the air that is released outside.
At this point I'll fill the exchanging box with another gas different from oxygen, nitrogen for example that presents inert characteristics.
I'll repeat this procedure at least twice, so that I'll be sure that there will be no air inside.
Now I can open the door between the exchanging box and the glove box and carry the instruments inside.
Working with the glove box, on the other hand, isn't so easy because of the huge gloves that aren't so confortable specially if you have to work with little dimensions!
Anyway we just need practice! :)
P.s. Glove box is also useful if we are working with toxic gasses. In this case we want that what is inside stays inside, so all the procedure described should be performed before exchanging materials from the glove box to the environment. Same thing but in the opposite way! :)
domenica 23 gennaio 2011
Anaerobic water - Back to the lab! :)
¡plɹoʍ ollǝɥ
prof: "Frantcieska, you should prepare an anaerobic solution for tomorrow..."
me aka FRANCESCA!!!: "Of course, ready for tomorrow!"
In the meanwhile in my mind a huuuuge "How???" echoed...
Soon i realized that's not such a difficult work!
Of course an equipped lab is necessary! ;)
The formal protocol says that the first step is boiling the water.
According to some recent study this step doesn't influence the results. So we just need a nitrogen flow from the bottom of the bottle. But why???
How can a flow of nitrogen eliminate the oxygen molecules?
That's pretty cool! :)
Henry's law:
The solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. So if the nitrogen concentration in the air above the water is high, the oxygen in the water will try to spread in the air to set a new equilibrium between partial pressions inside and outside the liquid.
Generally nitrogen is used because of its low solubility in water and specially because it is an inert gas.
P.S. Check this link! :)
prof: "Frantcieska, you should prepare an anaerobic solution for tomorrow..."
me aka FRANCESCA!!!: "Of course, ready for tomorrow!"
In the meanwhile in my mind a huuuuge "How???" echoed...
Soon i realized that's not such a difficult work!
Of course an equipped lab is necessary! ;)
The formal protocol says that the first step is boiling the water.
According to some recent study this step doesn't influence the results. So we just need a nitrogen flow from the bottom of the bottle. But why???
How can a flow of nitrogen eliminate the oxygen molecules?
That's pretty cool! :)
Henry's law:
At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
The solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. So if the nitrogen concentration in the air above the water is high, the oxygen in the water will try to spread in the air to set a new equilibrium between partial pressions inside and outside the liquid.
Generally nitrogen is used because of its low solubility in water and specially because it is an inert gas.
Mr William Henry (1775-1836) -Cool sideburns!
The analyte of our solution has to be added during the process, so when nitrogen is spreading.
The analyte of our solution has to be added during the process, so when nitrogen is spreading.
Of course it's necessary using particular stops to close the bottle. I used rubber stops with an aluminium cover.
That's all folk! :)
P.S. Check this link! :)
domenica 16 gennaio 2011
Polyhydroxyalkanoates 1.0
Dear beautiful folk!
I have just finished a course in Rome about Biomaterials and Biopolymers, cool stuff! :)
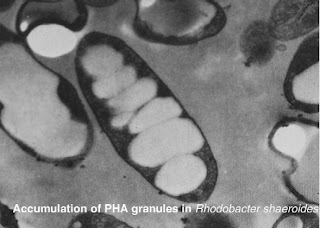
This is my report about Polyhydroxyalkanoates aka PHAs.
-SORRY IT'S IN ITALIAN-
I suggest also this article about PHAs:
Robert H. Marchessault Robert W. Lenz. Bacterial polyesters: Biosynte-sis, biodegradable plastics and biotechnology. BioMacromolecules, 6:1–8,2005.
These white balls are our PHAs, say hi! :)
sabato 1 gennaio 2011
"Daddy, how old will I be in 2000?"
A weird and funny year is finished.
Rome and Amsterdam conquered my heart! :)
Even if I'm a bit opposed to a New Year's resolution, I wrote couple of nice wishes that I'd like to grant...
So that's my "To Do" list:
- Taking the Master degree
- Do not pinning each single Italian problem on Berlusconi
- Buying more high-heeled shoes
- Losing four kilos
- Improving my English
- Studying Greek
- Reading less "useless" books
- Learning to sew
- Meeting more gay people
- Changing hair color
- Attending "La Traviata"
Iscriviti a:
Post (Atom)