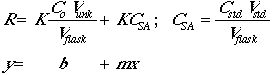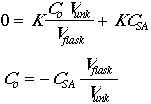Preparing a growth medium is as esay as following a recipe! :)
This is the colture medium I'm going to use with Desulfitobacteria hafniense DCB-2, D. hafniense TCE-1, D. chlororespirans, D. dehalogenans, D. PCE1.
This is the colture medium I'm going to use with Desulfitobacteria hafniense DCB-2, D. hafniense TCE-1, D. chlororespirans, D. dehalogenans, D. PCE1.
Pay attention to respect the right concentration and follow the protocol i've posted to prepare anaerobic solutions.
Starting solutions:
- M1= K2HPO4 (0.2 M)
- M2= NaH2PO4 * 2H2O (0.2 M)
- M3= Resazurine (0.5 g/L)
- M4= EDTA 500 (mg/L)
- M5=
- Vitamin H (50 mg/L)
- p-aminobenzoate (250 mg/L)
- Pantothenate (50 mg/L)
- Folic acid (20 mg/L)
- Lipoic acid (50 mg/L)
- Vitamin B6 (100 mg/L)
- Nicotinamide (550 mg/L)
- M6= Vitamin B1 (100 mg/L)
- M7= Vitamin B2 (50 mg/L)
- M8= Vitamin B12 (50 mg/L)
- M9= NH4HCO3 (114 mM) + NaHCO3 (906 mM)
- M10= Na2S * 9H2O (1M)
- M11= CaCl2 * H2O (30 mM) + MgCl2 * 6 H2O (20 mM)
- M12= Yeast extract (4%)
- M13= Na2SeO3 (6 mg/L)
All solutions were made in anaerobic water and flushed with N2 (except for M9 and M10 which contain volatile compounds).
Basic medium
- 21 ml M1
- 7 ml M2
- 1 ml M3
- 971 ml “sub-boiled” water
Cold solutions
- Cold A: in anaerobic, sterile bottle with 20 ml sterile demi-water added were: 1 ml M4, 1 ml M5, 1 ml M6, 1 ml M7, 1 ml M8, 0,5 ml M13.
- Cold B: 1 ml M10, 49 ml M9 filter-sterilised.
- Cold C: ~30 ml M11.
Completing the growing medium
To 18 ml basic medium (M1+M2+M3) add:
- 1 ml Cold B
- 0.5 ml Cold A
- 0.5 ml Cold C
- 1 ml 4% yeast extract
- Inoculum (10%)
 |
| Desulfitobacteria hafiense DCB-2 (University of California) |
So calculators in hand and good luck!
Soon to your screen the Best Pasta Ever recipe! :D
Soon to your screen the Best Pasta Ever recipe! :D